What is Galvanic Corrosion? How is Galvanic Corrosion Prevented?
Updated October 2023Galvanic corrosion, also called bimetallic corrosion, can occur between two different metals that are in electrical contact with each other in the presence of an electrolyte. When these conditions exist, one of the metals can be seen corroding much faster than the other. Carbon fiber is not a metal, but it is conductive like metal, which means metals in contact with carbon fiber can experience galvanic corrosion as well.

The airframe of the DarkAero 1 is built from a combination of carbon fiber composite and aerospace grade metal alloys, which meant its design had to incorporate strategies to prevent or at least mitigate against galvanic corrosion. Many tests were conducted to produce accelerated galvanic corrosion to derive solutions. Several of those solutions will be the focus of this article.
Before discussing the strategies to prevent galvanic corrosion, it is important to first understand the electrochemical mechanisms that cause it to occur. Galvanic corrosion requires three specific conditions that must exist simultaneously:
GALVANIC CORROSION CONDITIONS
1. Two dissimilar conductive materials are required, and they must have different electrode potentials.2. The two materials must be in electrical contact with each other. A simple way for this to occur is if they directly touch each other, but it could also happen if they are connected by a metal fastener or a wire.
3. An electrolyte must be present. Water or moisture is the most common electrolyte seen by the DarkAero 1, and it will be used as the electrolyte example in this article.
WHY DOES GALVANIC CORROSION OCCUR?
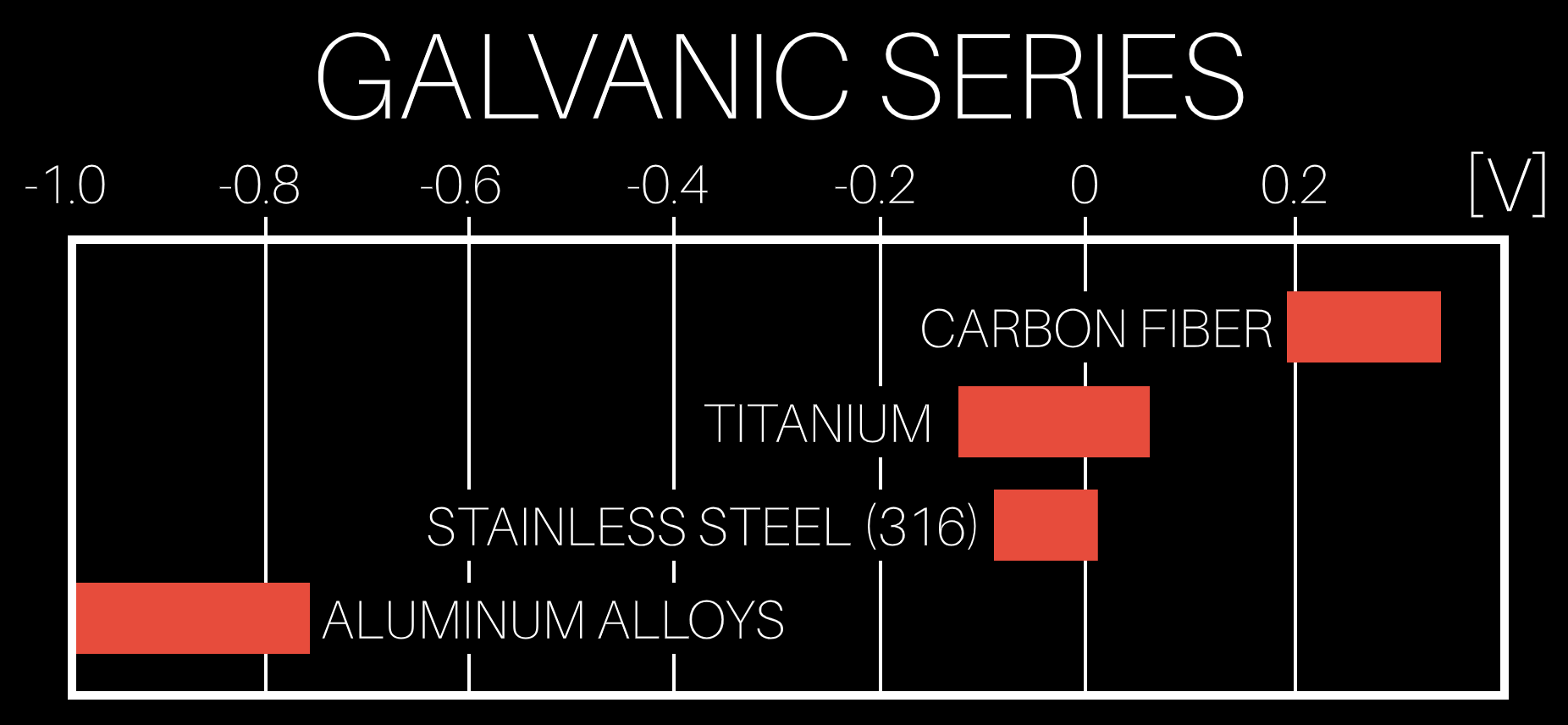 Earlier, the term electrode potential was mentioned. Metals and carbon fiber each have their own unique electrode potential, which is essentially a measure of how much a material wants to give up or receive electrons, and this is measured in volts. Scientists have measured these different potentials and organized them into the galvanic series. Materials that are further apart from one another in electrode potential, and thus further apart in the galvanic series, have a higher potential for galvanic corrosion when they come in contact. Carbon fiber and aluminum are far apart in the galvanic series, so they have a high potential for galvanic corrosion if they are used together.
Earlier, the term electrode potential was mentioned. Metals and carbon fiber each have their own unique electrode potential, which is essentially a measure of how much a material wants to give up or receive electrons, and this is measured in volts. Scientists have measured these different potentials and organized them into the galvanic series. Materials that are further apart from one another in electrode potential, and thus further apart in the galvanic series, have a higher potential for galvanic corrosion when they come in contact. Carbon fiber and aluminum are far apart in the galvanic series, so they have a high potential for galvanic corrosion if they are used together.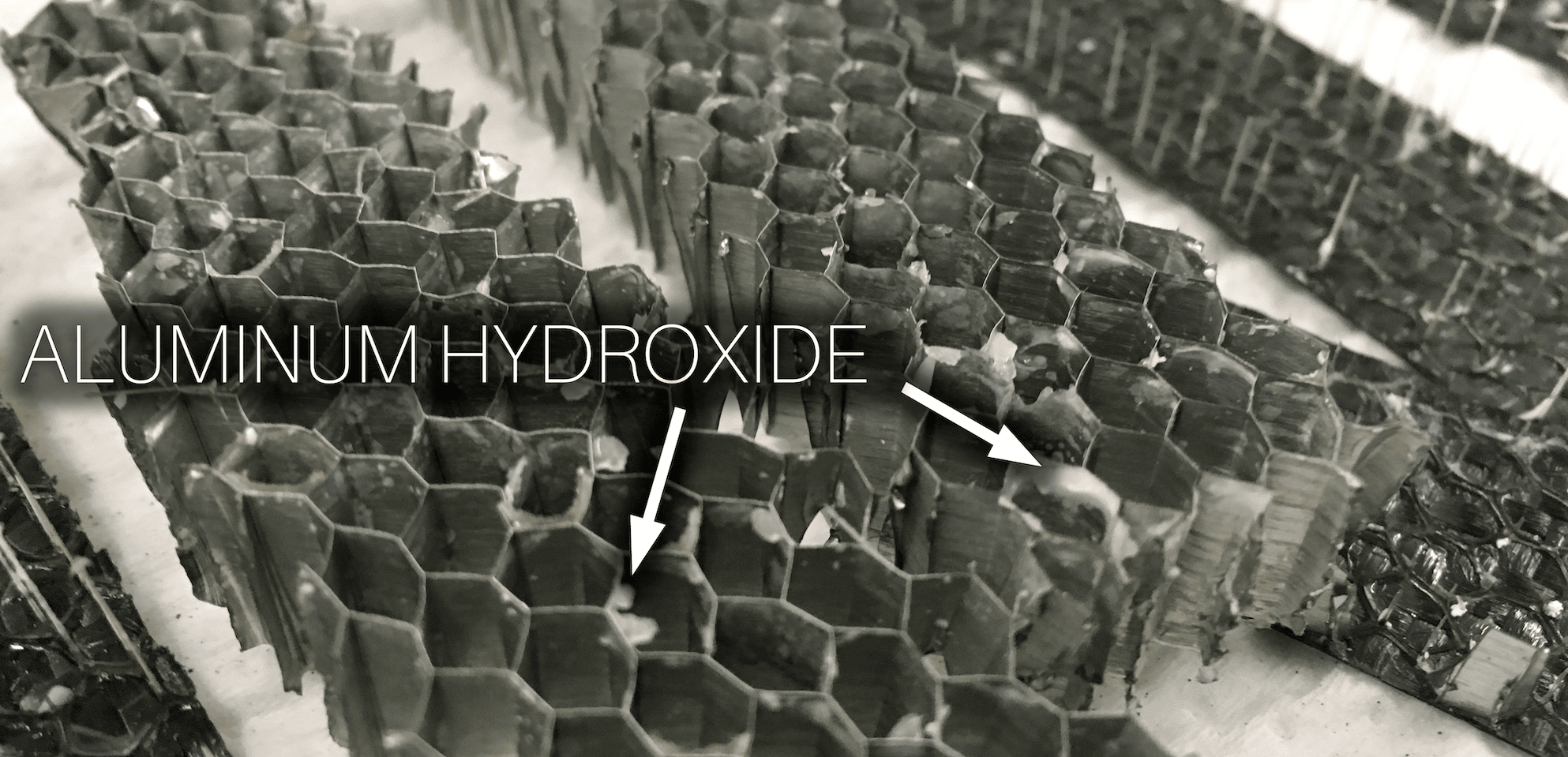 When all three galvanic corrosion conditions exist, a galvanic cell or battery is formed. In a galvanic cell made from aluminum and carbon fiber in the presence of water, the aluminum behaves as the anode in the cell, giving up electrons, and the carbon fiber acts as the cathode in the cell, taking electrons. The aluminum giving up its electrons is an oxidation reaction, which causes the aluminum to corrode. The water acts as a path for ion migration between the anode and cathode and prevents a charge build up that would otherwise stop the reaction. The end result is that the aluminum gets converted into aluminum hydroxide, which initially looks like a clear jelly on the aluminum, but it can dry out to a white solid.
When all three galvanic corrosion conditions exist, a galvanic cell or battery is formed. In a galvanic cell made from aluminum and carbon fiber in the presence of water, the aluminum behaves as the anode in the cell, giving up electrons, and the carbon fiber acts as the cathode in the cell, taking electrons. The aluminum giving up its electrons is an oxidation reaction, which causes the aluminum to corrode. The water acts as a path for ion migration between the anode and cathode and prevents a charge build up that would otherwise stop the reaction. The end result is that the aluminum gets converted into aluminum hydroxide, which initially looks like a clear jelly on the aluminum, but it can dry out to a white solid.HOW IS GALVANIC CORROSION PREVENTED?
With galvanic corrosion and its mechanisms now defined, what are some strategies to prevent it? A few ideas may already have come to mind based on the information shared so far. Recall that three conditions must exist for galvanic corrosion to occur. The key to prevention lies in eliminating any one of these conditions. Below are some of the ways that it is prevented on the DarkAero 1.Method 1: Use Compatible Metals
 One of the simplest ways to prevent galvanic corrosion is to use materials that are compatible with each other. A good example of this is the titanium firewall of the DarkAero 1. Titanium has a very small difference in electrode potential relative to carbon fiber so it can be used in direct contact with carbon fiber without significant risk of galvanic corrosion.
One of the simplest ways to prevent galvanic corrosion is to use materials that are compatible with each other. A good example of this is the titanium firewall of the DarkAero 1. Titanium has a very small difference in electrode potential relative to carbon fiber so it can be used in direct contact with carbon fiber without significant risk of galvanic corrosion.The fasteners that hold the ignition coils to the firewall of the DarkAero 1 are stainless steel and pass through the carbon fiber firewall structure, which brings them in contact with carbon fiber. Stainless steel fasteners are used in this area given their low difference in electrode potential with carbon fiber. Their location on the firewall also keeps them away from significant amounts of moisture exposure, which further helps minimize the risk of galvanic corrosion in this application.
Alternatively, carbon fiber panels can incorporate non-conductive hard points made from materials like phenolic, FR4, or cured solid epoxy. This allows metal fasteners for mounting hardware to pass through the panel while also keeping the fasteners and hardware electrically isolated from the carbon fiber.
Method 2: Isolate Metal With Glass Fiber
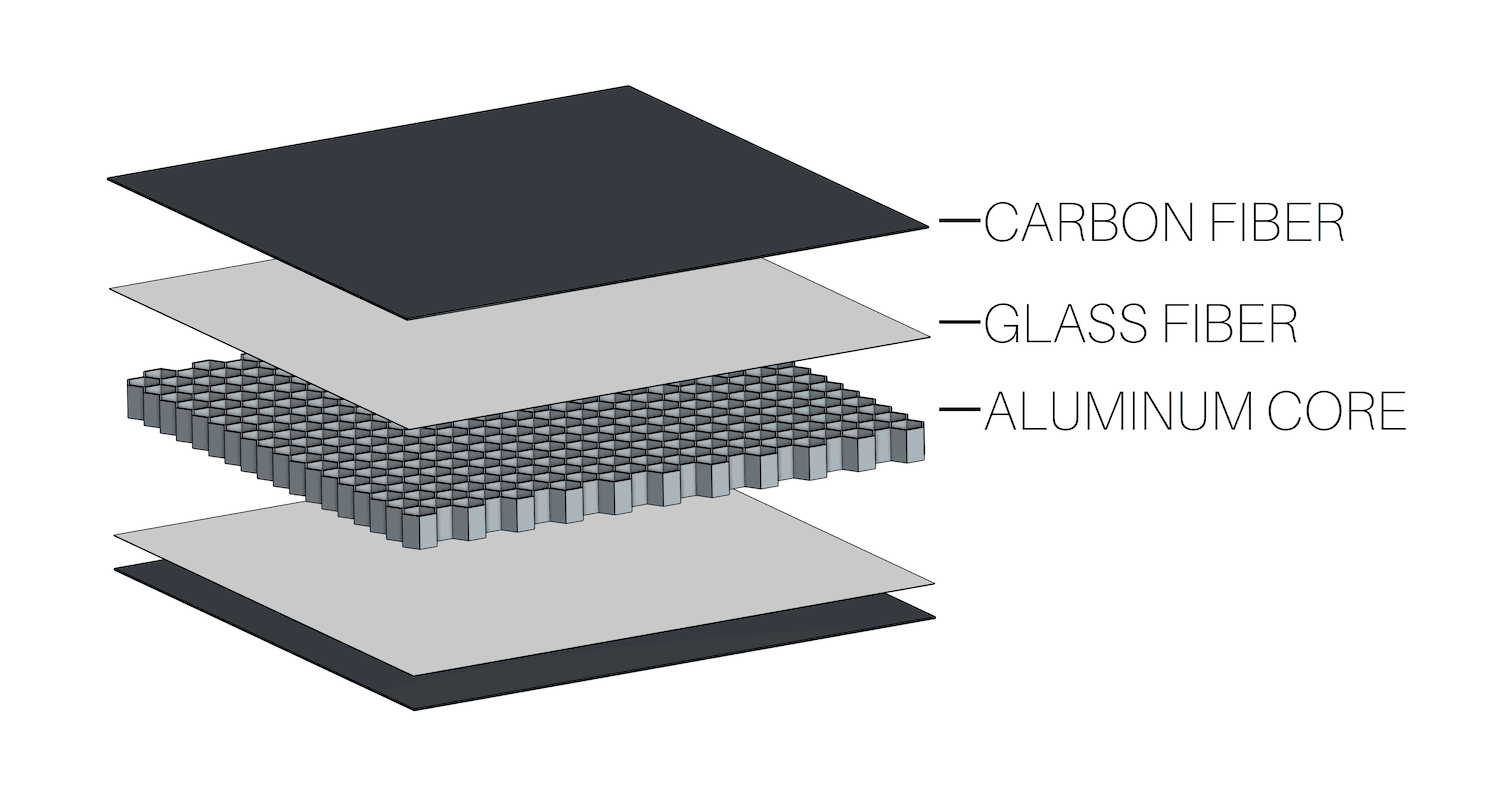 Another simple method used to prevent galvanic corrosion on the DarkAero 1 involves electrically isolating metals from carbon fiber using a layer of glass fiber. Many of the bulkheads in the DarkAero 1 prototype were made from carbon fiber skinned panels that contain an aluminum honeycomb core. This combination could experience galvanic corrosion, so a thin layer of glass fiber was installed between the carbon fiber skin and the aluminum honeycomb core to isolate the carbon fiber from the aluminum. The glass fiber layer is non-conductive, which prevents electrical contact between the carbon fiber and aluminum and therefore prevents galvanic corrosion. The diagram above shows an exploded view of the panel construction.
Another simple method used to prevent galvanic corrosion on the DarkAero 1 involves electrically isolating metals from carbon fiber using a layer of glass fiber. Many of the bulkheads in the DarkAero 1 prototype were made from carbon fiber skinned panels that contain an aluminum honeycomb core. This combination could experience galvanic corrosion, so a thin layer of glass fiber was installed between the carbon fiber skin and the aluminum honeycomb core to isolate the carbon fiber from the aluminum. The glass fiber layer is non-conductive, which prevents electrical contact between the carbon fiber and aluminum and therefore prevents galvanic corrosion. The diagram above shows an exploded view of the panel construction.Method 3: Isolate Metal With Paint
A method of electrically isolating carbon fiber from metal can also be seen on the aft electronics plate in the DarkAero 1. The carbon fiber plate was coated with several layers of paint, which acts as a non conductive barrier between the carbon fiber and any metal. The key is to paint the carbon fiber and not the metal component. A painted aluminum bracket that becomes chipped or scratched would create exposed aluminum and become a localized region of accelerated corrosion.Method 4: Isolate Metal With Adhesive
 Carbon fiber and metal can also be isolated from each other using adhesive. This solution can be seen with the Click Bond fasteners used on the DarkAero 1. The thin layer of non-conductive adhesive that holds the metal Click Bonds in place also prevents them from making electrical contact with the carbon fiber they are bonded to. Additionally, the Click Bonds themselves are made from stainless steel, which has a smaller difference in electrode potential relative to carbon fiber compared to aluminum and carbon fiber. This low electrode potential difference along with the layer of adhesive essentially eliminates any possibility of galvanic corrosion.
Carbon fiber and metal can also be isolated from each other using adhesive. This solution can be seen with the Click Bond fasteners used on the DarkAero 1. The thin layer of non-conductive adhesive that holds the metal Click Bonds in place also prevents them from making electrical contact with the carbon fiber they are bonded to. Additionally, the Click Bonds themselves are made from stainless steel, which has a smaller difference in electrode potential relative to carbon fiber compared to aluminum and carbon fiber. This low electrode potential difference along with the layer of adhesive essentially eliminates any possibility of galvanic corrosion.Method 5: Use Non-Conductive Materials
 Although the organic shape of the cooling inlets on the engine of the DarkAero 1 would make carbon fiber a favorable material choice, a non-conductive thermoplastic was chosen instead to eliminate the possibility of galvanic corrosion with the aluminum plenums the inlets are attached to.
Although the organic shape of the cooling inlets on the engine of the DarkAero 1 would make carbon fiber a favorable material choice, a non-conductive thermoplastic was chosen instead to eliminate the possibility of galvanic corrosion with the aluminum plenums the inlets are attached to.One other non-metal material selection example is the use of aramid honeycomb core rather than aluminum honeycomb core in the carbon fiber faced sandwich panels used in the DarkAero 1. While the fuselage bulkheads use aluminum honeycomb cores, the internal structures of the wings, horizontal stabilizer, and vertical stabilizer are made from panels that use aramid core, which eliminates the possibility of galvanic corrosion. Aramid honeycomb core will be used for all panel structures in the DarkAero 1 once it enters production.
There are other options to prevent galvanic corrosion that have not been implemented in the DarkAero 1 but may prove useful in other applications.
Method 6: Seal Out Moisture
One additional option is to prevent electrolytes (moisture) from contacting the metal and carbon fiber by completely sealing off the assembly of the two materials. This approach could be pursued if the two materials can not be isolated. It would require both materials to be completely encapsulated in some sort of coating to keep all moisture out. This would work in theory but would be potentially challenging to keep sealed and difficult to verify the seal is maintained throughout service.Method 7: Do Nothing
Another approach to galvanic corrosion is to take no action. For example, consider an assembly with a low service life like a model rocket designed for only a handful of launches. In the lifespan of the rocket, it may be acceptable to make the body of the rocket from a carbon fiber tube and bolt on some aluminum guide fins without experiencing any noticeable issues from galvanic corrosion.More discussion on galvanic corrosion and how to prevent it can be found in the video below:
Video: What Is Galvanic Corrosion and How Do We Prevent It on the DarkAero 1
Learn More
If you’d like to learn more about how to consistently make high-quality composite parts and get hands-on experience working with carbon fiber, consider enrolling in one of our courses:Aerospace Composites Course
Aerospace Mold Making Course
The courses are structured as a mix of both classroom lessons and hands-on demonstrations in the shop. Composite material fundamentals, design methods, material and process selection, and design for manufacturability are covered in class. Examples specific to student applications are used to immediately apply what is learned. Online versions of both courses are available as well at the links below:
Aerospace Composites Online Course
Aerospace Mold Making Online Course
